Each element has one or more isotopes are unstable nucleus will experience radioactive decay, causing the release core particles or electromagnetic radiation. Radioactivity can occur when the core radius is very large compared to the radius of the strong force (only works at distances of about 1 femtometer). Forms of radioactive decay is the most common are:
Alpha Decay
 |
| Image from Wikipedia |
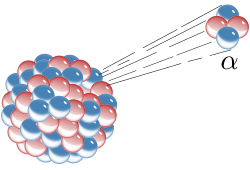
When an atom emits an alpha particle in alpha decay, the atom's mass number decreases by four due to the loss of the four nucleons in the alpha particle. The atomic number of the atom goes down by exactly two, as a result of the loss of two protons – the atom becomes a new element. Examples of this sort of nuclear transmutation are when uranium becomes thorium, or radium becomes radon gas, due to alpha decay. Alpha particles are commonly emitted by all of the larger radioactive nuclei such as uranium, thorium, actinium, and radium, as well as the transuranic elements. Unlike other types of decay, alpha decay as a process must have a minimum-size atomic nucleus that can support it.The process of emitting an alpha sometimes leaves the nucleus in an excited state, with the emission of a gamma ray removing the excess energy.(http://en.wikipedia.org/wiki/Alpha_particle)
Beta Decay
In the beta decay of particles, there are three ways that will be passed to the event are:
In the beta decay of particles, there are three ways that will be passed to the event are:
 |
| Image from Wikipedia |
β− decay (electron emission). An unstable atomic nucleus with an excess of neutrons may undergo β− decay, where a neutron is converted into a proton, an electron and an electron-type antineutrino (the antiparticle of the neutrino)
β+ decay (positron emission). Unstable atomic nuclei with an excess of protons may undergo β+ decay, also called positron decay, where a proton is converted into a neutron, a positron and an electron-type neutrino.
Interaction with other matter. Of the three common types of radiation given off by radioactive materials, alpha, beta and gamma, beta has the medium penetrating power and the medium ionising power. Although the beta particles given off by different radioactive materials vary in energy, most beta particles can be stopped by a few millimeters of aluminium. Being composed of charged particles, beta radiation is more strongly ionising than gamma radiation. When passing through matter, a beta particle is decelerated by electromagnetic interactions and may give off bremsstrahlung x-rays. (http://en.wikipedia.org/wiki/Beta_particle)
Gamma Decay/ Gamma Radiation
 |
| Image from Wikipedia |
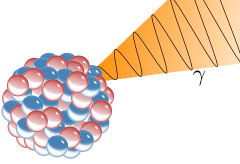
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency and therefore energy. Gamma rays are ionizing radiation and are thus biologically hazardous. Gamma rays are classically produced by the decay from high energy states of atomic nuclei (gamma decay), but also in many other ways. Natural sources of gamma rays on Earth include gamma decay from naturally-occurring radioisotopes such as potassium-40, and also as a secondary radiation from various atmospheric interactions with cosmic ray particles. Some rare terrestrial natural sources that produce gamma rays that are not of a nuclear origin, are lightning strikes and terrestrial gamma-ray flashes, which produce high energy emissions from natural high-energy voltages.
Gamma rays from radioactive gamma decay are produced alongside other forms of radiation such as alpha or beta, and are produced after the other types of decay occur. The mechanism is that when a nucleus emits an α or β particle, the daughter nucleus is usually left in an excited state. It can then move to a lower energy state by emitting a gamma ray, in much the same way that an atomic electron can jump to a lower energy state by emitting a photon.Gamma decay from excited states may also follow nuclear reactions such as neutron capture, nuclear fission, or nuclear fusion. (http://en.wikipedia.org/wiki/Gamma_ray)
when the nucleus emits an alpha particle, it loses two protons and two neutrons. When the atomic nucleus emits beta particles, neutrons to protons changes. When the atomic nuclei emit gamma rays that reconfigure the core, itself into a high state of energy.
All radioactive elements will decay within a certain period, the decay of the light it emits alpha, beta, and gamma in order to achieve a stable level or state. For example:


nice
ReplyDeleteNice Post.... :)
ReplyDelete